Abstract
Objective: To delineate the distribution, clinical characteristics and prognostic features of different subtypes of Castleman disease based on the CDCN criteria. To describe the first-line treatment choice for iMCD in China before the era of IL-6-directed therapy.
Methods: This was a national, observational, retrospective study which enrolled consecutive patients with CD diagnosed at 40 large Chinese institutions from 2000 to 2021. Patients were classified into different CD subtypes according to the CDCN criteria. The primary outcomes were the distribution of different CD subtypes, descriptions of demographic and clinical characteristics, treatment options and overall survival.
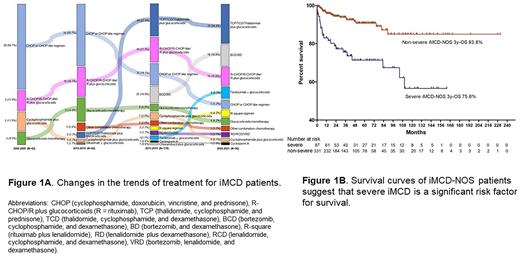
Results: A total of 1634 CD patients (unicentric CD(UCD), n=903; multicentric CD(MCD), n=731) were enrolled. Among UCDs, hyaline-vascular (HV) was the major pathological subtype, and there were 162(17.9%) patients with an MCD-like inflammatory state (UCD-MIS). Among the MCD patients, there were 12 HHV-8-related MCD patients and 719 HHV-8-negative MCD patients (asymptomatic MCD (aMCD), n=139; iMCD, n=580). Of the 580 iMCD patients, 41(7.1%) were classified as having iMCD-TAFRO, while the others were classified as having iMCD-NOS. Among the iMCD patients with first-line treatment information, a trend from pulse combination chemotherapy toward a continuous approach was noticed (Fig 1A). Survival analysis revealed significant differences between different subtypes, and the estimated 3-year overall survival rates for UCD, iMCD-NOS and iMCD-TAFRO patients were 98.0%, 89.6% and 65.7%, respectively. Among the iMCD-NOS patients, the multivariate Cox regression model identified 'severe iMCD' (HR=4.540; 95% CI 1.888-10.915, p=0.001) as the only independent risk factor for death (Fig 1B). The estimated 3-year overall survival rates for severe and nonsevere iMCD-NOS patients were 93.8% and 75.6%, respectively.
Conclusion: This study depicts a whole picture of CD subtype distribution, treatment options and survival information in China and validates the rationality of the concept of severe iMCD proposed by the CDCN.
Disclosures
Chen:Takeda Pharmaceutical Company Limited: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal